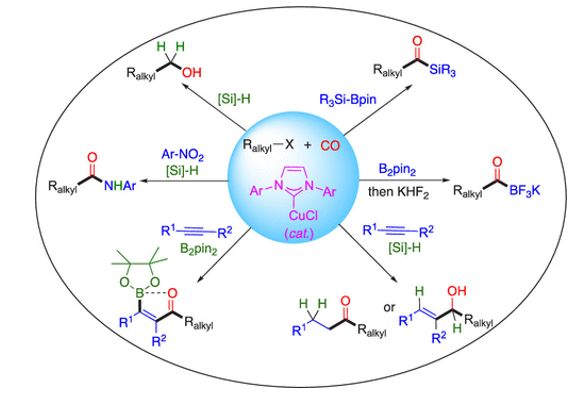
Carbonylation reactions incorporate CO into organic feedstocks to make value-added chemicals. The most successful examples of homogeneous catalysis in industry to date are carbonylation reactions such as Rh-catalyzed alkene hydroformylation and Rh- or Ir-catalyzed alcohol carbonylation that are performed industrially on scales of millions of tons per year. Additionally, Pd-catalyzed carbonylative cross coupling reactions are mainstay methods for synthesizing complex organic structures. Recently, motivated by both sustainability considerations and the possibility of discovering new reactivity, there has been a surge in research on non-precious metal catalysts for carbonylation. In this context, we began studying Cu-catalyzed carbonylation reactions in 2017, which ultimately seeded the growth of an entire sub-field to which we contributed a significant body of work. These reactions employ simple Cu(I) catalysts to promote multicomponent coupling reactions between CO, alkyl halides, and (pro-)nucleophiles. The one-pot, three- or four-component coupling reactions enable rapid increases in molecular complexity from simple building blocks. A hallmark of these reactions is that they proceed by radical carbonylation pathways: the Cu(I) catalyst engages the alkyl halide in single-electron transfer, generating an alkyl radical that captures CO to form acyl radical prior to rebounding with the catalyst for reductive elimination. Due to the lack of any alkylcopper intermediate, unwanted side reactions like 𝛽-hydride elimination that plague Pd-catalyzed coupling reactions of C(sp3)-hybridized electrophiles are bypassed. In recent and ongoing work, we have expanded the scope of Cu-catalyzed carbonylation using visible light photocatalysis.
This project is currently funded by DOE through grant DE-SC0021055

Department of Chemistry
