Portable energy storage has impacted society dramatically in the last two decades, and continued development of such technologies will be critical to support renewable energy economies.
An exciting technology to improve upon state-of-art Li-ion batteries is the Li metal battery, which has failed to commercialize due to safety concerns associated with the Li metal anodes.
Thus, a vibrant research area is identification of electrolyte additives and other methods for influencing the solid-electrolyte interphase (SEI) of Li metal in ways that maintain favorable electron and ion transport properties while preventing uncontrolled electrolyte decomposition reactions and hazardous dendrite deposits.
Dimolybdenum complexes with quadruple bonds exhibit reversible redox chemistry, reductive stability, and synthetic modularity.
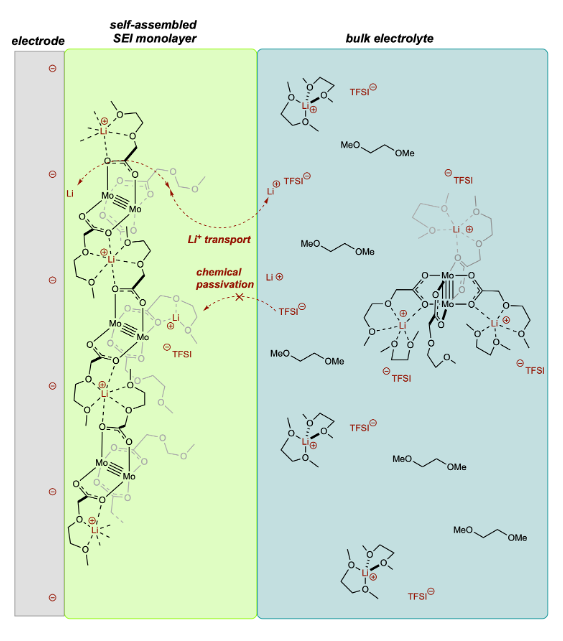
We recently discovered that the quadruply-bonded paddlewheel complex, Mo2(mea)4 [mea = 2-(methoxy)ethoxyacetate] binds multiple Li + ions in the second coordination sphere using its hemi-crown ether tails, producing cationically charged coordination polymers that adsorb to electrode surface under working conditions.
The coated electrodes are found to be chemically passivated toward electrolyte decomposition even at extreme applied potentials (< 0 V vs. Li) while maintaining conductivity and promoting reversible Li plating and stripping.
Ongoing and future work includes optimizing impact of this first-in-class organometallic electrolyte additive in Li metal battery settings, as well as development of second-generation derivatives with improved behavior in Li metal battery settings. We also plan to extend this platform to “beyond Li” technologies such as Na and Mg metal batteries.
Check back soon for our first publication!
